For detailed guidance, please refer to:
- Seidler AL, Hunter K, Cheyne S, Ghersi D, Berlin J, Askie L. A guide to prospective meta-analysis. BMJ 2019;367:l5342. [Pubmed]
- The Cochrane Handbook for Systematic Reviews of Interventions, chapter 22.
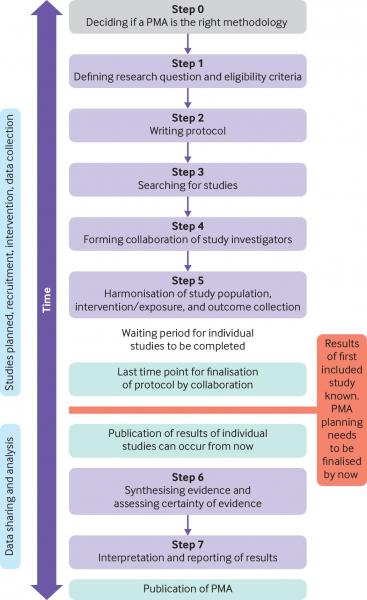
As outlined in the figure1 below, the general steps taken in order to conduct a PMA include the following:
Step 0: deciding if a PMA is the right methodology.
- PMA methodology should be considered for a high priority research question for which new studies are expected to emerge and limited previous evidence exists.
- If sufficient evidence is available, no further studies and no PMA should be planned.
- If evidence is available, but is insufficient for clinical decision making, a nested PMA should be considered.
Step 1: defining the research question and the eligibility criteria
- Research questions should be formed and defined in a similar way as for non-PMA systematic reviews, with detailed guidance available in the Cochrane Handbook for Systematic Reviews of Interventions.2
Step 2: writing the protocol
- Use the preferred reporting items for systematic reviews and meta-analyses extensions for protocols (PRISMA-P).3
- Ensure that the final protocol is registered on PROSPERO.
Step 3: searching for studies
- Studies eligible for inclusion in a PMA should be identified in a similar way as for non-PMA systematic reviews, with detailed guidance available in the Cochrane Handbook for Systematic Reviews of Interventions.2
Step 4: forming a collaboration of study investigators
Step 5: harmonisation of included study population, intervention/exposure, and outcome collection
- Once a collaboration of planned or ongoing studies is formed, the investigators can harmonise study components to facilitate meta-analysis and interpretation.
Step 6: synthesising the evidence and assessing certaintly of evidence
- Data are synthesised and included studies appraised using validated tools, certaintly of evidence can be assessed with the grading of recommendations assessment, development and evaluation (GRADE) approach.4
Step 7: interpretation and reporting of results
- No PMA-specific reporting guidelines exist, but researchers should follow the PRISMA statement5 or PRISMA-IPD6 where applicable.
References:
1. Seidler, A.L., Hunter, K.E., Cheyne, S., Ghersi, D., Berlin, J.A. and Askie, L., 2019. A guide to prospective meta-analysis. bmj, 367. [Pubmed]
2. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available here.
3. Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P. and Stewart, L.A., 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews, 4(1), pp.1-9. [Pubmed]
4. Guyatt, G., Oxman, A.D., Akl, E.A., Kunz, R., Vist, G., Brozek, J., Norris, S., Falck-Ytter, Y., Glasziou, P., DeBeer, H. and Jaeschke, R., 2011. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology, 64(4), pp.383-394. [Pubmed]
5. Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G. and Prisma Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 6(7), p.e1000097. [Pubmed]
6. Stewart, L.A., Clarke, M., Rovers, M., Riley, R.D., Simmonds, M., Stewart, G. and Tierney, J.F., 2015. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. Jama, 313(16), pp.1657-1665. [Pubmed]
