MuSE-Evidence Synthesis
Do you want to contribute to guidance for engaging with multiple interest-holders throughout the evidence synthesis process? If so, please complete this short form so we can be in touch when we are ready to collect survey responses. The survey will ask about whether you think your interest-holder group (see below) should be engaged for each of the different steps of an evidence synthesis.
We are developing guidance for engaging with 11 categories of interest-holders throughout the evidence synthesis process.
Our 11 interest-holder categories are:
- Patients/consumers, caregivers, and patient groups
- Payers/funders of research
- Payers and purchasers of health services (e.g. those who pay for or reimburse health-related interventions, including insurers, individuals with deductibles, others, and those entities responsible for underwriting the cost of care, such as employers and governments)
- Publishers/peer review editors (those involved in the knowledge translation of evidence syntheses, e.g. peer-review editors, scientific publishers, science writers)
- Policy-makers (e.g. governments and professional associations, those involved in the regulatory processes of drugs and health devices)
- Principal investigators and all members of the research team (e.g. researchers conducting studies that may or may not be relevant to the review)
- Product makers (e.g. drug, natural products and/or device manufacturers)
- Producers and commissioners of evidence syntheses(e.g. institutions and organizations that commission, develop, or implement guideline development procedures)
- Program managers (e.g. managers/directors/administrators and individuals who plan, lead, oversee, or deliver any program that provides public health, community services, or clinical care (e.g. budgeting, hiring, staffing, organizing, coordinating, reporting). These individuals may be health care providers but are not on the point of care delivering health care related to the program of interest (e.g. overseeing an immunisation program but not delivering vaccinations)
- Providers (individuals and/or organizations providing care, such as nurses, physicians, pharmacists, community-based workers)
- Public (e.g. communities or general members of the population or community, excluding patients, caregivers, and health professionals, living or working with the condition of interest)
Check our Open Science Framework page for our review series' protocols:
Tugwell P, Welch V, Magwood O, Todhunter-Brown A, Akl EA, et al. Protocol for the development of guidance for collaborator and partner engagement in health care evidence syntheses. Systematic Reviews. 2023;12:134.
In August, 2021, our team, led by Peter Tugwell, Vivian Welch, and Alex Todhunter-Brown, received funding for a 4 year project to:
- Identify, map, and synthesize qualitative and quantitative findings related to engagement in evidence syntheses
- Explore perspectives on how engagement in evidence syntheses promotes health equity
- Develop equity-oriented guidance on methods for engagement in evidence synthesis
- Develop guidance on methods for evaluating engagement in evidence syntheses
- Develop a guideline for reporting engagement in evidence syntheses (Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension).
The protocol for this project was published in August, 2023: Protocol for the development of guidance for collaborator and partner engagement in health care evidence syntheses. Systematic Reviews. 2023;12:134.
The project includes 4 sequential steps: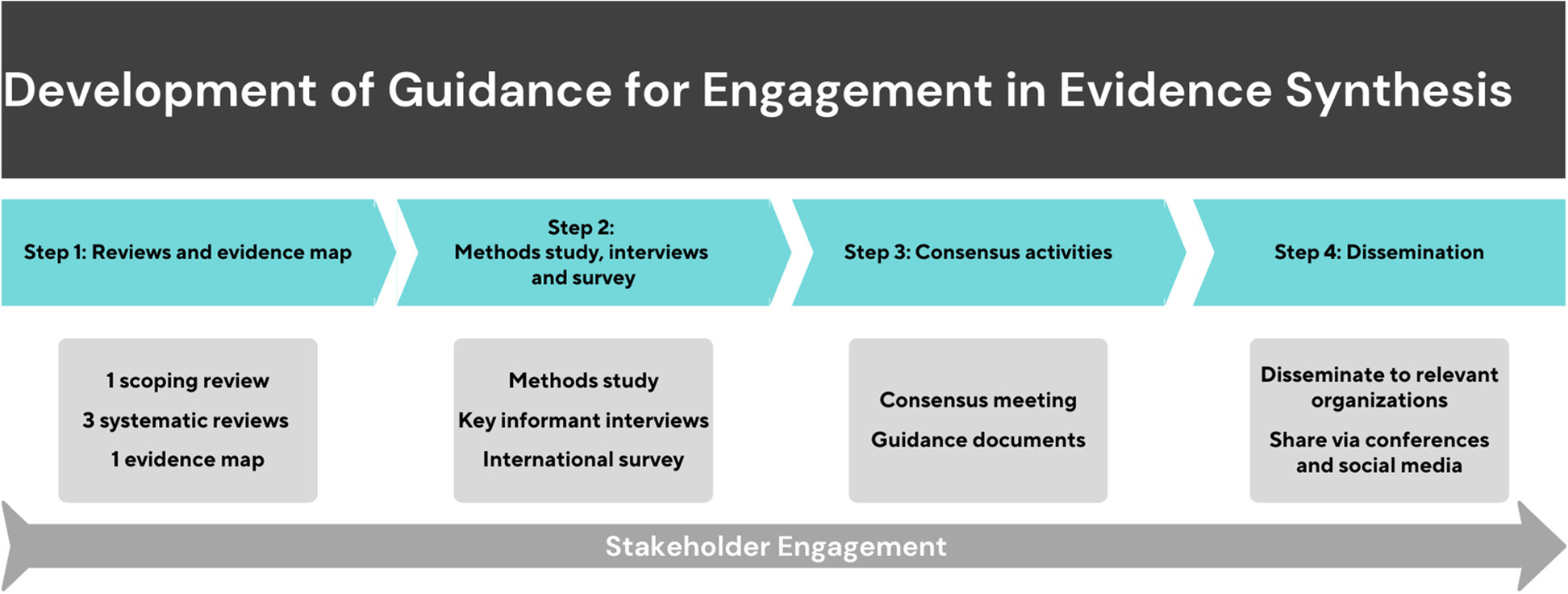
Interested in learning more about this project? Contact Jennifer Petkovic or follow us on Twitter
